
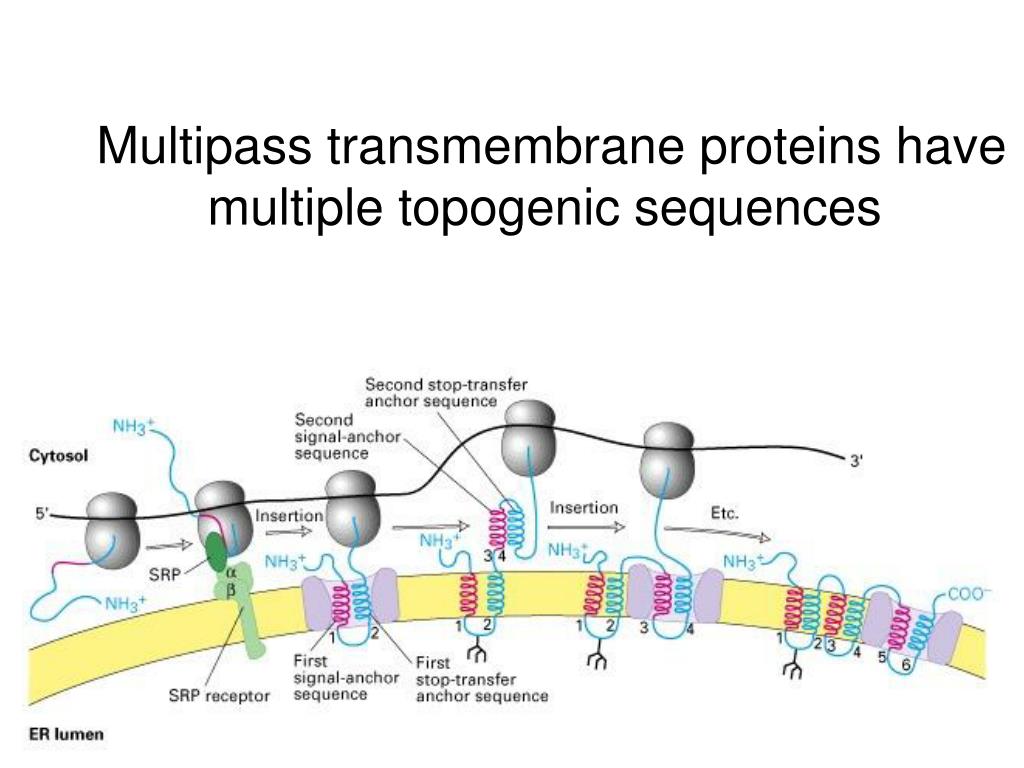
In multipass proteins with N-terminus in the cytosol (type IV-A), the TM helix sequence that first emerges from the ribosome (the one closest to the N-terminus) functions in the same way as the start transfer sequence (signal-anchor sequence) of the type II protein, which interacts with SRP to target the protein to the translocon, opens the translocon channel, and allow growing polypeptide chain to pass through the channel. Since the adjacent helices are in opposite orientations, insertion of multipass membrane proteins can be viewed as an alternation of insertion of a type I helix and insertion of a type II helix, which is dictated by the TM helix sequences that alternatingly act as start-transfer signals (signal anchor sequences) and stop-transfer signals (stop-transfer anchor sequences). type IV proteins) contain more than one TM \(\alpha\) helices and traverse the membrane multiple times. Eventually, the hydrophic N- and C- termini are on the luminal and the cytosolic face, respectively. The C-terminus continues to be synthesised and loops out on the cytosolic side of the membrane. This segment is the TM helix of the nascent protein, and it acts as a stop-transfer signal (or “stop-transfer anchor sequence”) by triggering the lateral opening of Sec61 \(\alpha\) and thus allowing this helix to move into the membrane. After cleavage, translation continues and the newly synthesised sequence is threaded through the channel and enters the ER lumen, until another hydrophobic segment is encountered. Signal peptidase cleaves off the signal peptide once it recognises a specific sequence on the C-terminal end of the signal peptide. The sequence following the signal peptide displaces the plug and inserts into the central channel, resulting in the conformation shown in Figure 2.1. Sec61 \(\alpha\) opens the lateral gate, and the signal peptide fits into the exposed hydrophobic bindng pocket. The signal sequence is recognised by SRP, causing the ribosome-nascent peptide complex to be targeted to the Sec61 translocon. Type I membrane proteins, like soluble proteins to be translocated into the ER lumen, contain an N-terminal ER-targeting signal sequence to be cleaved.
The verticle opening allows elongation of the nascent peptide chain through the central pore, and the lateral opening allows attachment of signal sequences and exit of TM helices into the membrane (Figure 1.1). The channel can also open laterally by hinging apart the two 5-helix bundles to expose a hydrophobic binding pocket for signal sequences and/or TM helices of the nascent peptide. The central channel through Sec61 \(\alpha\) is sealed by a plug made of a helical peptide that only opens during traslocation. The Sec61 translocon is made up of 3 subunits: Sec61 \(\alpha\), an integral membrane protein composed of two 5-helix bundles that constitutes the central channel and two smaller proteins, Sec61 \(\beta\) and Sec61 \(\gamma\). GTP replaces GDP on both proteins, and they are ready to initiate another round of ER targeting. Once the nascent chain has left SRP and enters the translocon, the two GTP molecules are hydrolysed, causing dissociation of SRP and its receptor. The GTPase activity is inhibited by signal peptide binding. When their GTP-bound forms associate with each other, they form two GTPase active sites. SRP and SRP receptor each has a GTP binding site. Recognition by SRP not only serves to target the ribosome-nascent protein to the ER but also prevents exposure of the hydrophobic segment to the aqueous environment. This segment can either be a pure ER-targeting signal sequence or be the first TM helix itself (see next section). Usually it is the first hydrophobic segment that is recognised by SRP. Meanwhile, the Alu domain blocks the elongation factor 2 (eEF2) binding site on the ribosome, pausing translation. This cleft binds to a hydrophobic segment of the nascent peptide as soon as it emerges from the exit tunnel. The M domain contains a cleft whose inner surface is lined by methionine and other hydrophobic side chains. The M and Alu domains of SRP are critical for its functions. SRP is a ribonucleoprotein particle made up of 6 proteins bound to a 300-nucleotide RNA acting as a scaffold. The common co-translationally inserted membrane proteins are targeted to the Sec61 translocon on the ER membrane via the cytosolic signal recognition particle (SRP) and the SRP receptor near the translocon. 1 Targeting the Protein to the ER Membrane: SRP and the Sec61 Translocon


 0 kommentar(er)
0 kommentar(er)
